how to draw newman projections for cyclic compounds
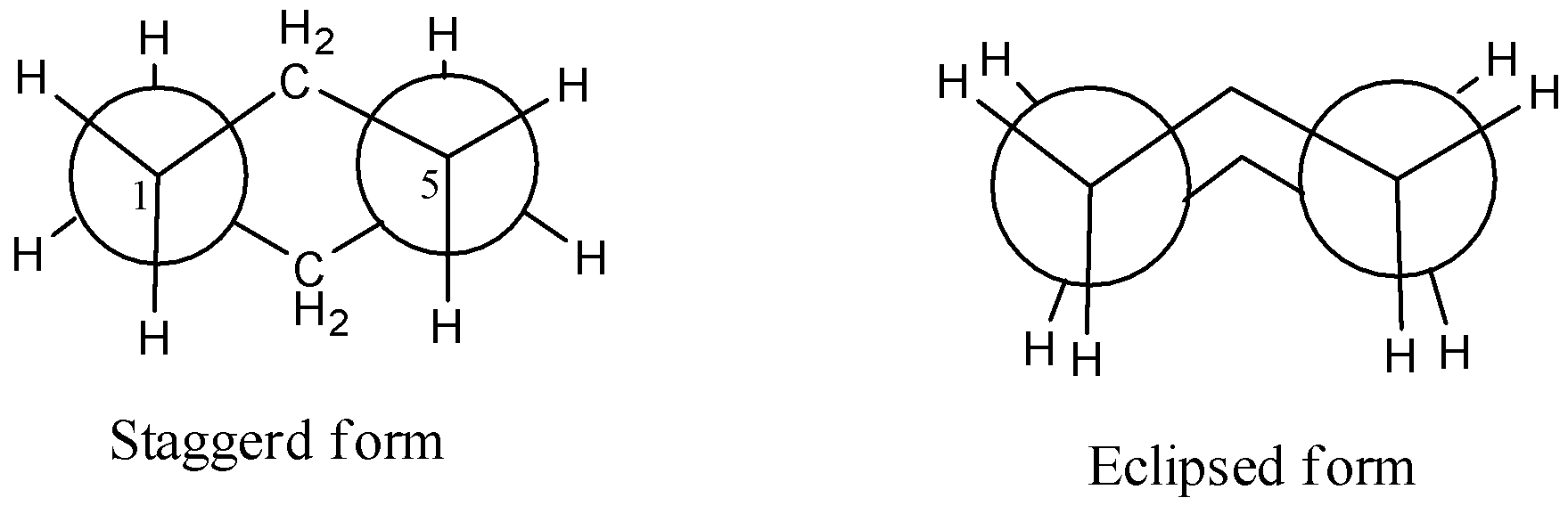
For example in butane the most common axis for Newman projection is the C2-C3 axis. 1955 32 344 For many years the Fischer projection formulas have been used to represent the stereochemistry of molecules with multiple asymmetric centers.

Ring Flip Of Chair Conformations With Practice Problems Chemistry Steps
The higher the atomic number the higher the priority.

. Converting Cyclohexane Chair Conformations to Double Newman ProjectionsNeed help with Orgo. Add the groups here they are H atoms to the bonds extending from the Newman projections. Lets draw one for 1R2R4S-4-chloro-2-iodo-1-methylcyclohexane.
They were initially proposed by Emil Fischer for making it easier to draw the structures of compounds containing multiple chirality centers with the main idea of not having to draw the wedge and dash lines for every single chiral centerThis is especially applicable and used mostly for drawing. Give each atom connected to the chiral center a priority based on its atomic number. Redraw the molecule such that the wedged and dashed groups on the left-most stereocenter are oriented downward.
How to draw R and S absolute configurations from a name Occasionally you will have to draw absolute configurations from a name. The Newman projection is viewed on a line from the. And not just draw them but convert between them and line-bond aka bond-line or zig-zag structures.
A Newman projection is a way to take a snapshot of what a molecule looks like at a particular moment in time from a different angle than were used to. So based on this bromine gets priority one the oxygen gets priority two the methyl carbon is the third and the hydrogen is the lowest priority-four. Locate all chiral centers 4 different groups at sp3 atoms and assign the priorities of the groups at each chiral atom.
Now what I want to do is draw two Newman projections and both of them will involve-- well actually Ill draw four but youll see what Im talking about in a second. You can draw them close together just connect both of them to the top CH2 the back of the chair and the bottom CH2 bottom of the chair. Newman projections 6 Melvin Spencer Newman Newman M.
The following strategy should prove helpful. Write out a two dimensional structure from the name. Our goal in this article is to draw and analyze the Newman projection shown on the.
Basically if you were to look down that bond what you would see is that your big groups this ethyl group here and this ethyl group here would be on opposite sides of these carbons because as you can tell if you were to draw your dotted line they. Join the two diagrams with the front carbon C-6 at the top and the rear carbon C-3 at the bottom. ChemDraw can analyze molecules drawn in Newman projections to the same extent as it can analyze molecules in other representations.
- Among the different types of projections the Newmann projection is written in a way where the front carbon is represented as a dot and back carbon as a circle and the cyclic molecules are written in the form of their conformations. Moc members get access to over 1500. It is similar to a Newman projection but this type of projection shows the carbon-carbon bond at the middle of the molecule unlike a Newman projection.
Draw Newman projection for the molecule below from the perspective indicated. For each of the following draw the best most stable and worst least stable Newman projection relative to the bond indicated in each question. For chair cyclohexane imagine looking down the two bonds that form the seat of the chair C2-C3 and C5-C6.
Download my free guide 10 S. Cyclic Compounds Modelling Exercise 1. The most stable conformations will be staggered conformations with the largest groups ANTI to each other.
3 pts CH CH 2. When drawing Newman projections look at the molecule from a. Draw the lowest energy conformations of the molecules shown below and identify all the gauche-butane.
Basically all we have to do is create two separate Newman projections and link them together through two different carbons. Newman projections focus on any two carbons and the groups coming off them in a molecule by shifting the view from which the molecule is visualized. These formulas are adequate if one is dealing only with the classical aspects of stereoisomerism.
In the video below Ive distilled the process for doing this and I guide you through two examples. What is Sawhorse Projection. ChemDraw also supports Fischer projections and Haworth projections but those are only mentioned for comparison in this article.
So the first Newman projection Im going to start at that carbon right over there. Once youve mastered the art of naming alkanes sooner or later in organic chemistry youre going to have to draw Newman projections. Generally the main axis or axis of interest or carbon-carbon bond of interest will be a central carbon bond in your molecule.
Therefore the stick group should be oriented upward. Redraw the molecule such that all four groups present on each stereocenter are shown. For the boat form repeat the process.
You can draw Newman projections for cyclic molecules. Both of these have gauche conformations. Sawhorse projection is the display of a molecule from an angle rather than the side-on projection.
Fischer projections are just another way of drawing compounds contacting chirality centers. For example we can even use it to study cyclic molecules. For the Newman projection you drew indicate if there are any eclipsed bonds or gauche butane interactions.
A Haworth projection is a common way of writing a structural formula to represent the cyclic structure of monosaccharides with a simple three-dimensional perspective. How to draw newman projections organicchemexplained newman projections check out the interactive tutorial on for this one we will need to be able to draw newman projections as well as name the molecule using the rules of iupac how to convert a molecule drawn as a newman projection into a line diagram. And over here once again is the one carbon two three four five six.
A trans or E configuration for a double bond can exist in cyclic compounds but a large ring is necessary or the strain in the compound is very high. Of course you can draw a Newman projection down any bond axis but the C2-C3 axis is the most beneficial to view in a Newman projection. Cyclic alkenes with 3 - 7 atoms in the ring are fixed in the cis or Z structure.
Organic chemistry and especially biochemistry are the areas of chemistry that use the Haworth projection the most. Newman Projections More Practice Answer Key I. Now cyclohexane molecules are having different conformations and among that chair form is a more stable conformer.
With Newman projections youre allowed to rotate around that bond in a three-dimensional way. The steps on changing Wedge-Dash Notation into a Newman Projection will be given first. Draw side-by-side projections of the C1-C2 and C5-C4 bonds.
We actually use what amounts to two Newman projections stuck together and we call it a double Newman.

Cyclohexane Conformation Chair Conformation And Axial Equatorial Stability Ryosuke University

Ashley S Biology Study Guides Refreshers And Reviews For The Biologist In You Organic Chemistry Organic Chemistry Study Chemistry

Pin On Aldehydes And Ketones Practice Problems
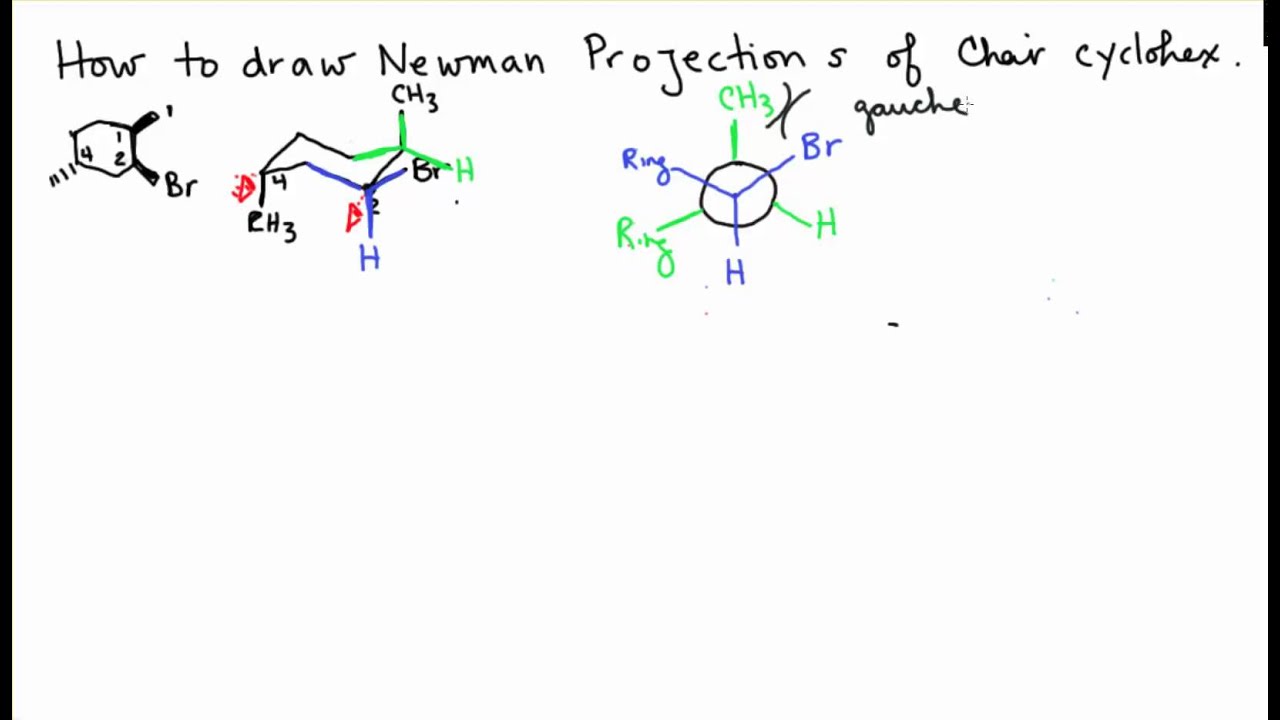
Double Newman Projection Chair Cyclohexane Youtube
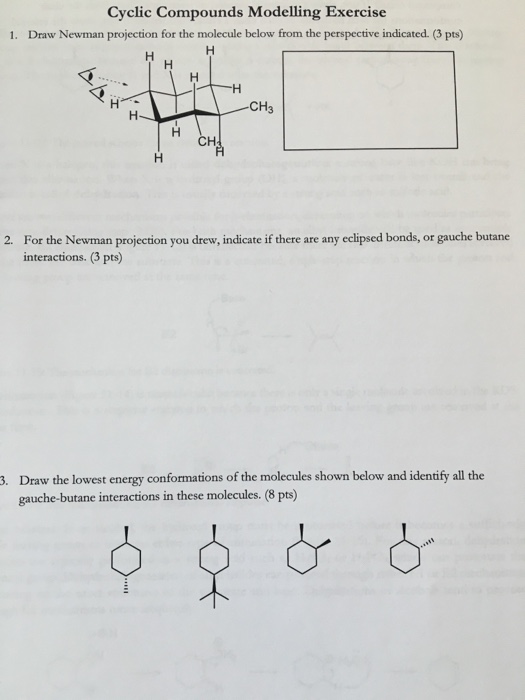
Solved Cyclic Compounds Modelling Exercise 1 Draw Newman Chegg Com

Cyclohexane Chair Conformation To Double Newman Projection Youtube

Solved Part Ii Conformations Of Cyclic Compounds A Chegg Com

Orgo 2 Practice Exam Q2 Basicity Of Aromatic Compounds Practice Exam Organic Chemistry Chemistry
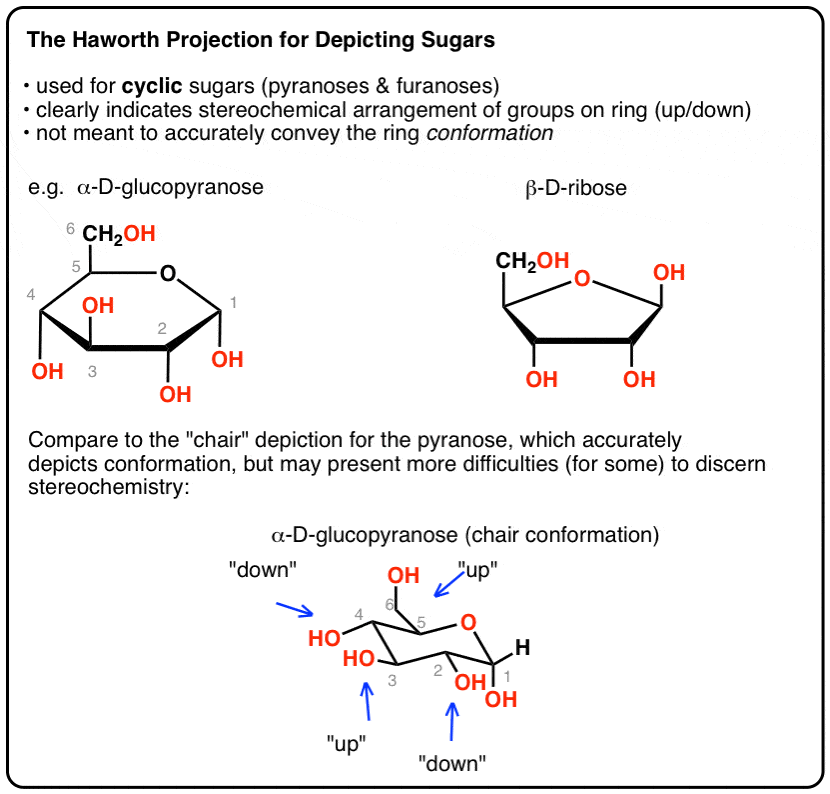
The Haworth Projection Master Organic Chemistry

Primary Secondary And Tertiary Amines Organic Chemistry Chemistry Secondary
How Can I Draw A Newman Projection For Cyclohexane Class 12 Chemistry Cbse

Aromaticity Means Cyclic Planar Conjugated And Huckel S Rule For A Stable Pi System This Includes Multi Re Organic Chemistry Chemistry Organic Chemistry Study

Dibal Reduction Reaction Of Ester Or Nitrile To Aldehyde Organic Chemistry Chemistry Reduction

Orgo 2 Practice Exam Q2 Basicity Of Aromatic Compounds Practice Exam Organic Chemistry Chemistry

Chair Conformation And Ring Flips Youtube

Conformational Analysis Newman Projections Ring Strain Cyclohexane Conformations Ppt Download

D Glucose And L Glucose Are Enantiomers While D Glucose And D Mannose Are Epimers Organic Chemistry Study Chemistry D Glucose

